C
Structure and Nomenclature of Hydrocarbons
The Saturated Hydrocarbons, or Alkanes
When you drive up to the pump at some gas stations you are faced with a variety of choices.
You can buy "leaded" gas or different forms of "unleaded" gas that have different octane numbers. As you filled the tank, you might wonder, "What is 'leaded' gas, and why do they add lead to gas?" Or, "What would I get for my money if I bought premium gas, with a higher octane number?"
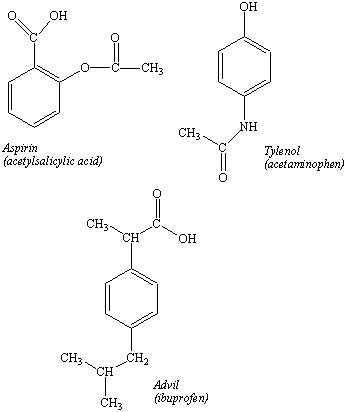
You then stop to buy drugs for a sore back that has been bothering you since you helped a friend move into a new apartment. Once again, you are faced with choices (see the figure below). You could buy aspirin, which has been used for almost a hundred years. Or Tylenol, which contains acetaminophen. Or a more modern pain-killer, such as ibuprofen. While you are deciding which drug to buy, you might wonder, "What is the difference between these drugs?," and even, "How do they work?"
You then drive to campus, where you sit in a "plastic" chair to eat a sandwich that has been wrapped in "plastic," without worrying about why one of these plastics is flexibile while the other is rigid. While you're eating, a friend stops by and starts to tease you about the effect of your diet on the level of cholesterol in your blood, which brings up the questions, "What is cholesterol?" and "Why do so many people worry about it?"
Answers to each of these questions fall within the realm of a field known as organic chemistry. For more than 200 years, chemists have divided materials into two categories. Those isolated from plants and animals were classified as organic, while those that trace back to minerals were inorganic. At one time, chemists believed that organic compounds were fundamentally different from those that were inorganic because organic compounds contained a vital force that was only found in living systems.
| For more than 200 years, chemists have divided
materials into two categories.
Those isolated from plants and animals were classified as organic Those that trace back to minerals were inorganic Chemists believed that organic compounds were different because they contained a vital force that was only found in living systems. in 1828, when Friederich Wohler synthesized urea from inorganic starting materials
|
The first step in the decline of the vital force theory occurred in 1828, when Friederich Wohler synthesized urea from inorganic starting materials. Wohler was trying to make ammonium cyanate (NH4OCN) from silver cyanate (AgOCN) and ammonium chloride (NH4Cl). What he expected is described by the following equation.
AgOCN(aq) + NH4Cl(aq)
![]() AgCl(s) + NH4OCN(aq)
AgCl(s) + NH4OCN(aq)
The product he isolated from this reaction had none of the properties of cyanate compounds. It was a white, crystalline material that was identical to urea, H2NCONH2, which could be isolated from urine.
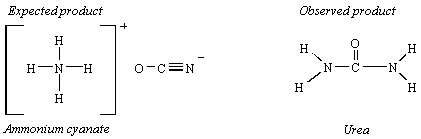
Neither Wohler nor his contemporaries claimed that his results disproved the vital force theory. But his results set in motion a series of experiments that led to the synthesis of a variety of organic compounds from inorganic starting materials. This inevitably led to the disappearance of "vital force" from the list of theories that had any relevance to chemistry, although it did not lead to the death of the theory, which still had proponents more than 90 years later.
If the difference between organic and inorganic compounds isn't the presence of some
mysterious vital force required for their synthesis, what is the basis for distinguishing
between these classes of compounds? Most compounds extracted from living organisms contain
carbon. It is therefore tempting to identify organic chemistry as the chemistry of carbon.
But this definition would include compounds such as calcium carbonate (CaCO3),
as well as the elemental forms of carbon ![]() diamond
and graphite
diamond
and graphite![]() that are clearly inorganic.
We will therefore define organic chemistry as the chemistry of compounds that contain
both carbon and hydrogen.
that are clearly inorganic.
We will therefore define organic chemistry as the chemistry of compounds that contain
both carbon and hydrogen.
| We will define organic chemistry as the chemistry of compounds that contain both carbon and hydrogen.
|
Even though organic chemistry focuses on compounds that contain carbon and hydrogen, more than 95% of the compounds that have isolated from natural sources or synthesized in the laboratory are organic. The special role of carbon in the chemistry of the elements is the result of a combination of factors, including the number of valence electrons on a neutral carbon atom, the electronegativity of carbon, and the atomic radius of carbon atoms (see the table below).
The Physical Properties of Carbon
| Electronic configuration | 1s2 2s2 2p2 | |
| Electronegativity | 2.55 | |
| Covalent radius | 0.077 nm |
Carbon has four valence electrons ![]() 2s2
2p2
2s2
2p2![]() and it must
either gain four electrons or lose four electrons to reach a rare-gas configuration. The
electronegativity of carbon is too small for carbon to gain electrons from most elements
to form C4- ions, and too large for carbon to lose electrons to form C4+
ions. Carbon therefore forms covalent bonds with a large number of other elements,
including the hydrogen, nitrogen, oxygen, phosphorus, and sulfur found in living systems.
and it must
either gain four electrons or lose four electrons to reach a rare-gas configuration. The
electronegativity of carbon is too small for carbon to gain electrons from most elements
to form C4- ions, and too large for carbon to lose electrons to form C4+
ions. Carbon therefore forms covalent bonds with a large number of other elements,
including the hydrogen, nitrogen, oxygen, phosphorus, and sulfur found in living systems.
Because they are relatively small, carbon atoms can come close enough together to form
strong C=C double bonds or even C![]() C
triple bonds. Carbon also forms strong double and triple bonds to nitrogen and oxygen. It
can even form double bonds to elements such as phosphorus or sulfur that do not form
double bonds to themselves.
C
triple bonds. Carbon also forms strong double and triple bonds to nitrogen and oxygen. It
can even form double bonds to elements such as phosphorus or sulfur that do not form
double bonds to themselves.
Several years ago, the unmanned Viking spacecraft carried out experiments designed to search for evidence of life on Mars. These experiments were based on the assumption that living systems contain carbon, and the absence of any evidence for carbon-based life on that planet was presumed to mean that no life existed. Several factors make carbon essential to life.
These factors provide an almost infinite variety of potential structures for organic compounds, such as vitamin C shown in the figure below.
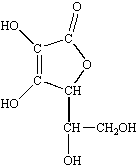
No other element can provide the variety of combinations and permutations necessary for life to exist.
The Saturated Hydrocarbons, or Alkanes
Compounds that contain only carbon and hydrogen are known as hydrocarbons. Those that contain as many hydrogen atoms as possible are said to be saturated. The saturated hydrocarbons are also known as alkanes.
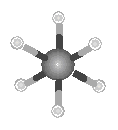
The simplest alkane is methane: CH4. The Lewis structure of methane can be generated by combining the four electrons in the valence shell of a neutral carbon atom with four hydrogen atoms to form a compound in which the carbon atom shares a total of eight valence electrons with the four hydrogen atoms.
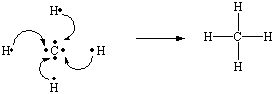
Methane is an example of a general rule that carbon is tetravalent; it
forms a total of four bonds in almost all of its compounds. To minimize the repulsion
between pairs of electrons in the four C![]() H
bonds, the geometry around the carbon atom is tetrahedral, as shown in the figure below.
H
bonds, the geometry around the carbon atom is tetrahedral, as shown in the figure below.

|
Use the fact that carbon is usually tetravalent to predict the formula of ethane, the alkane that contains two carbon atoms. |
The alkane that contains three carbon atoms is known as propane, which has the formula C3H8 and the following skeleton structure.
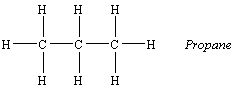
The four-carbon alkane is butane, with the formula C4H10.
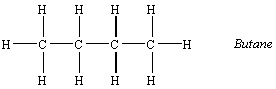
The names, formulas, and physical properties for a variety of alkanes with the generic formula CnH2n+2 are given in the table below. The boiling points of the alkanes gradually increase with the molecular weight of these compounds. At room temperature, the lighter alkanes are gases; the midweight alkanes are liquids; and the heavier alkanes are solids, or tars.
The Saturated Hydrocarbons, or Alkanes
| Name | Molecular Formula |
Melting Point (oC) |
Boiling Point (oC) |
State at 25oC |
||||
| methane | CH4 | -182.5 | -164 | gas | ||||
| ethane | C2H6 | -183.3 | -88.6 | gas | ||||
| propane | C3H8 | -189.7 | -42.1 | gas | ||||
| butane | C4H10 | -138.4 | -0.5 | gas | ||||
| pentane | C5H12 | -129.7 | 36.1 | liquid | ||||
| hexane | C6H14 | -95 | 68.9 | liquid | ||||
| heptane | C7H16 | -90.6 | 98.4 | liquid | ||||
| octane | C8H18 | -56.8 | 124.7 | liquid | ||||
| nonane | C9H20 | -51 | 150.8 | liquid | ||||
| decane | C10H22 | -29.7 | 174.1 | liquid | ||||
| undecane | C11H24 | -24.6 | 195.9 | liquid | ||||
| dodecane | C12H26 | -9.6 | 216.3 | liquid | ||||
| eicosane | C20H42 | 36.8 | 343 | solid | ||||
| triacontane | C30H62 | 65.8 | 449.7 | solid |
The alkanes in the table above are all straight-chain hydrocarbons, in which the carbon atoms form a chain that runs from one end of the molecule to the other. The generic formula for these compounds can be understood by assuming that they contain chains of CH2 groups with an additional hydrogen atom capping either end of the chain. Thus, for every n carbon atoms there must be 2n + 2 hydrogen atoms: CnH2n+2.
Because two points define a line, the carbon skeleton of the ethane molecule is linear, as shown in the figure below.
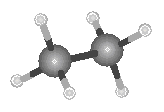
Because the bond angle in a tetrahedron is 109.5, alkanes molecules that contain three or four carbon atoms can no longer be thought of as "linear," as shown in the figure below.
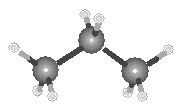 |
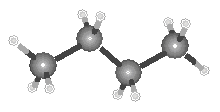 |
||
| Propane | Butane |
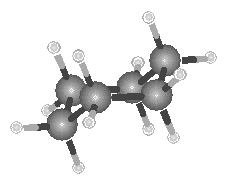
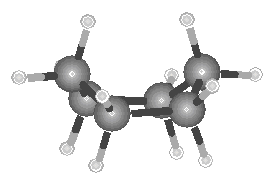
In addition to the straight-chain examples considered so far, alkanes also form branched structures. The smallest hydrocarbon in which a branch can occur has four carbon atoms. This compound has the same formula as butane (C4H10), but a different structure. Compounds with the same formula and different structures are known as isomers (from the Greek isos, "equal," and meros, "parts"). When it was first discovered, the branched isomer with the formula C4H10 was therefore given the name isobutane.
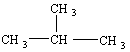 Isobutane
Isobutane
The best way to understand the difference between the structures of butane and isobutane is to compare the ball-and-stick models of these compounds shown in the figure below.
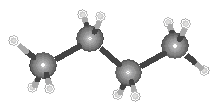 |
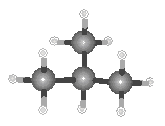 |
||
| Butane | Isobutane |
Butane and isobutane are called constitutional isomers because they literally differ in their constitution. One contains two CH3 groups and two CH2 groups; the other contains three CH3 groups and one CH group.
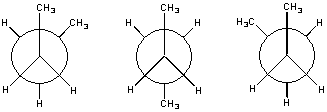
There are three constitutional isomers of pentane, C5H12. The first is "normal" pentane, or n-pentane.
![]()
A branched isomer is also possible, which was originally named isopentane. When a more highly branched isomer was discovered, it was named neopentane (the new isomer of pentane).
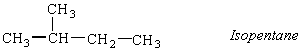
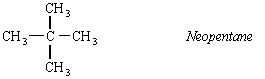
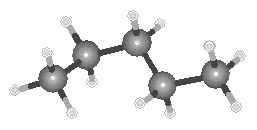
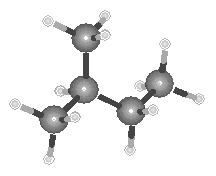
Ball-and-stick models of the three isomers of pentane are shown in the figure below.
 |
 |
||
| n-Pentane | Isopentane |
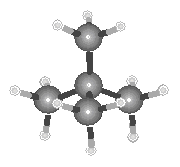 |
| Neopentane |
| Practice Problem 2: The following structures all have the same molecular formula: C6H14. Which of these structures represent the same molecule?
|
| Practice Problem 3: Determine the number of constitutional isomers of hexane, C6H14. |
There are two constitutional isomers with the formula C4H10, three isomers of C5H12, and five isomers of C6H14. The number of isomers of a compound increases rapidly with additional carbon atoms. There are over 4 billion isomers for C30H62, for example.
If the carbon chain that forms the backbone of a straight-chain hydrocarbon is long
enough, we can envision the two ends coming together to form a cycloalkane.
One hydrogen atom has to be removed from each end of the hydrocarbon chain to form the C![]() C bond that closes the ring. Cycloalkanes
therefore have two less hydrogen atoms than the parent alkane and a generic formula of CnH2n.
C bond that closes the ring. Cycloalkanes
therefore have two less hydrogen atoms than the parent alkane and a generic formula of CnH2n.
The smallest alkane that can form a ring is cyclopropane, C3H6,
in which the three carbon atoms lie in the same plane. The angle between adjacent C![]() C bonds is only 60�, which is very much
smaller than the 109.5� angle in a tetrahedron, as shown in the figure below.
C bonds is only 60�, which is very much
smaller than the 109.5� angle in a tetrahedron, as shown in the figure below.
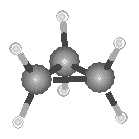
Cyclopropane is therefore susceptible to chemical reactions that can open up the three-membered ring.
Any attempt to force the four carbons that form a cyclobutane ring into a plane of
atoms would produce the structure shown in the figure below, in which the angle between
adjacent C![]() C bonds would be 90�.
C bonds would be 90�.
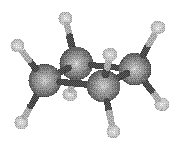
One of the four carbon atoms in the cyclobutane ring is therefore displaced from the plane of the other three to form a "puckered" structure that is vaguely reminiscent of the wings of a butterfly.
The angle between adjacent C![]() C bonds in
a planar cyclopentane molecule would be 108�, which is close to the ideal angle around a
tetrahedral carbon atom. Cyclopentane is not a planar molecule, as shown in the figure
below, because displacing two of the carbon atoms from the plane of the other three
produces a puckered structure that relieves some of the repulsion between the hydrogen
atoms on adjacent carbon atoms in the ring.
C bonds in
a planar cyclopentane molecule would be 108�, which is close to the ideal angle around a
tetrahedral carbon atom. Cyclopentane is not a planar molecule, as shown in the figure
below, because displacing two of the carbon atoms from the plane of the other three
produces a puckered structure that relieves some of the repulsion between the hydrogen
atoms on adjacent carbon atoms in the ring.
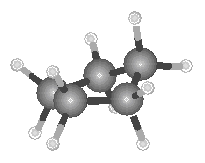
By the time we get to the six-membered ring in cyclohexane, a puckered structure can be formed by displacing a pair of carbon atoms at either end of the ring from the plane of the other four members of the ring. One of these carbon atoms is tilted up, out of the ring, whereas the other is tilted down to form the "chair" structure shown in the figure below.
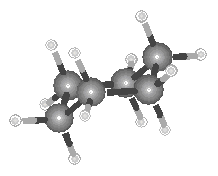
As one looks at the structure of the ethane molecule, it is easy to fall into the trap
of thinking about this molecule as if it was static. Nothing could be further from the
truth. At room temperature, the average velocity of an ethane molecule is about 500 m/s
![]() more than twice the speed of a Boeing 747.
While it moves through space, the molecule is tumbling around its center of gravity like
an airplane out of control. At the same time, the C
more than twice the speed of a Boeing 747.
While it moves through space, the molecule is tumbling around its center of gravity like
an airplane out of control. At the same time, the C![]() H and C
H and C![]() C bonds are vibrating
like a spring at rates as fast as 9 x 1013 s-1.
C bonds are vibrating
like a spring at rates as fast as 9 x 1013 s-1.
There is another way in which the ethane molecule can move. The CH3 groups
at either end of the molecule can rotate with respect to each around the C![]() C bond. When this happens, the molecule passes
through an infinite number of conformations that have slightly different
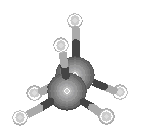
energies. The highest energy conformation corresponds to a structure in which the hydrogen
atoms are "eclipsed." If we view the molecule along the C
C bond. When this happens, the molecule passes
through an infinite number of conformations that have slightly different
energies. The highest energy conformation corresponds to a structure in which the hydrogen
atoms are "eclipsed." If we view the molecule along the C![]() C bond, the hydrogen atoms on one CH3
group would obscure those on the other, as shown in the figure below.
C bond, the hydrogen atoms on one CH3
group would obscure those on the other, as shown in the figure below.
The lowest energy conformation is a structure in which the hydrogen atoms are "staggered," as shown in the figure below.
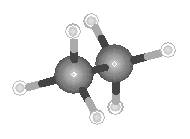
The difference between the eclipsed and staggered conformations of ethane are best
illustrated by viewing these molecules along the C![]() C bond, as shown in the figure below.
C bond, as shown in the figure below.
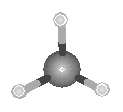 |
 |
||
| Eclipsed | Staggered |
The difference between the energies of these conformations is relatively
small, only about 12 kJ/mol. But it is large enough that rotation around the C![]() C bond is not smooth. Although the frequency
of this rotation is on the order of 1010 revolutions per second, the ethane
molecule spends a slightly larger percentage of the time in the staggered conformation.
C bond is not smooth. Although the frequency
of this rotation is on the order of 1010 revolutions per second, the ethane
molecule spends a slightly larger percentage of the time in the staggered conformation.
The different conformations of a molecule are often described in terms of Newman
projections. These line drawings show the six substituents on the C![]() C bond as if the structure of the molecule was
projected onto a piece of paper by shining a bright light along the C
C bond as if the structure of the molecule was
projected onto a piece of paper by shining a bright light along the C![]() C bond in a ball-and-stick model of the
molecule. Newman projections for the different staggered conformations of butane are shown
in the figure below.
C bond in a ball-and-stick model of the
molecule. Newman projections for the different staggered conformations of butane are shown
in the figure below.
Because of the ease of rotation around C![]() C bonds, there are several conformations of some of the cycloalkanes
described in the previous section. Cyclohexane, for example, forms both the
"chair" and "boat" conformations shown in the figure below.
C bonds, there are several conformations of some of the cycloalkanes
described in the previous section. Cyclohexane, for example, forms both the
"chair" and "boat" conformations shown in the figure below.
 |
 |
||
| Chair | Boat |
The difference between the energies of the chair conformation, in which the hydrogen atoms are staggered, and the boat conformation, in which they are eclipsed, is about 30 kJ/mol. As a result, even though the rate at which these two conformations interchange is about 1 x 105 s-1, we can assume that most cyclohexane molecules at any moment in time are in the chair conformation.
Common names such as pentane, isopentane, and neopentane are sufficient to differentiate between the three isomers with the formula C5H12. They become less useful, however, as the size of the hydrocarbon chain increases.
The International Union of Pure and Applied Chemistry (IUPAC) has developed a systematic approach to naming alkanes and cycloalkanes based on the following steps.
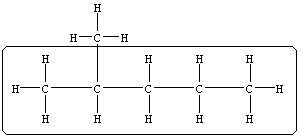
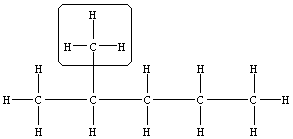
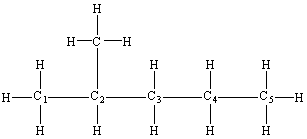
| Practice Problem 4: Name the following compound.
|
| Practice Problem 5: Name the following compound.
|
The Unsaturated Hydrocarbons: Alkenes and Alkynes
Carbon not only forms the strong C![]() C
single bonds found in alkanes, it also forms strong C=C double bonds. Compounds that
contain C=C double bonds were once known as olefins (literally, "to make an
oil") because they were hard to crystallize. (They tend to remain oily liquids when
cooled.) These compounds are now called alkenes. The simplest alkene has
the formula C2H4 and the following Lewis structure.
C
single bonds found in alkanes, it also forms strong C=C double bonds. Compounds that
contain C=C double bonds were once known as olefins (literally, "to make an
oil") because they were hard to crystallize. (They tend to remain oily liquids when
cooled.) These compounds are now called alkenes. The simplest alkene has
the formula C2H4 and the following Lewis structure.
![]()
The relationship between alkanes and alkenes can be understood by
thinking about the following hypothetical reaction. We start by breaking the bond in an H2
molecule so that one of the electrons ends up on each of hydrogen atoms. We do the same
thing to one of the bonds between the carbon atoms in an alkene. We then allow the
unpaired electron on each hydrogen atom to interact with the unpaired electron on a carbon
atom to form a new C![]() H bond.
H bond.
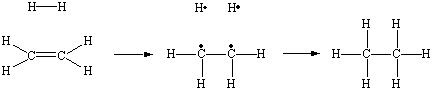
Thus, in theory, we can transform an alkene into the parent alkane by adding an H2 molecule across a C=C double bond. In practice, this reaction only occurs at high pressures in the presence of a suitable catalyst, such as piece of nickel metal.
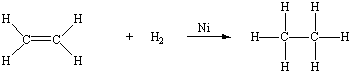
Because an alkene can be thought of as a derivative of an alkane from which an H2 molecule has been removed, the generic formula for an alkene with one C=C double bond is CnH2n.
Alkenes are examples of unsaturated hydrocarbons because they have fewer hydrogen atoms than the corresponding alkanes. They were once named by adding the suffix -ene to the name of the substituent that carried the same number of carbon atoms.
![]()
The IUPAC nomenclature for alkenes names these compounds as derivatives of the parent alkanes. The presence of the C=C double bond is indicated by changing the -ane ending on the name of the parent alkane to -ene.
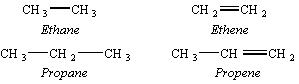
The location of the C=C double bond in the skeleton structure of the compound is indicated by specifying the number of the carbon atom at which the C=C bond starts.
![]()
The names of substituents are then added as prefixes to the name of the alkene.
| Practice Problem 6: Name the following compound.
|
Compounds that contain C![]() C triple bonds are called alkynes. These compounds have four
less hydrogen atoms than the parent alkanes, so the generic formula for an alkyne with a
single C
C triple bonds are called alkynes. These compounds have four
less hydrogen atoms than the parent alkanes, so the generic formula for an alkyne with a
single C![]() C
triple bond is CnH2n-2. The simplest alkyne has the formula C2H2
and is known by the common name acetylene.
C
triple bond is CnH2n-2. The simplest alkyne has the formula C2H2
and is known by the common name acetylene.
![]()
The IUPAC nomenclature for alkynes names these compounds as derivatives of the parent alkane, with the ending -yne replacing -ane.
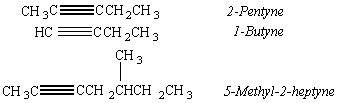
In addition to compounds that contain one double bond (alkenes) or one triple bond (alkynes), we can also envision compounds with two double bonds (dienes), three double bonds (trienes), or a combination of double and triple bonds.
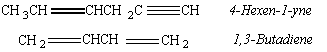
Organic Chemistry: Structure and Nomenclature of Hydrocarbons
Structure and Nomenclature of Hydrocarbons | Isomers | The Reactions of Alkanes, Alkenes, and Alkynes | Hydrocarbons | Petroleum and Coal | Chirality and Optical Activity
Periodic Table | Periodic Table | Glossary | Cool Applets
Gen Chem Topic Review | General Chemistry Help Homepage | Search: The general chemistry web site.