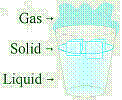STANDARD SET 6. Chemistry of Living
Systems
|
6. Principles of chemistry underlie
the functioning of biological systems. a. Carbon, because of its ability to
combine in many ways with itself and other elements, has a central role in the
chemistry of living organisms |
|
|
CARBON |
|
|
Of the naturally occurring elements, carbon
is probably the most important organic element. On the Periodic Table,
carbon is the first member of Group IV. Group IV members have four valence electrons,
so they can become stable by either losing or gaining four electrons.
Other groups will either lose or gain electrons, but with Group IV's
flexibility, these atoms, particularly carbon, tend to share electrons. Sharing electrons forms strong covalent
bonds between atoms. Group IV atoms can form four covalent bonds, one for
each electron. Remember that electrons have a negative charge. So these
electrons repel each other. For the bonds to become stable, they must lie
equal distance from each other. This arrangement forms a tetrahedron. A
tetrahedron is like a camera tripod, with the legs and camera stand having
equal lengths. Of the Group IV atoms, carbon forms the most stable covalent
bonds. Carbon
is also unique in that it can form very stable bonds with other carbon atoms.
It is this nature that forms the foundation for organic molecules. Proteins,
sugars, fats and genetic material is made with a carbon backbone, upon which
other elements are attached.
|
|
|
|
|
|
|
|
b. Living organisms are made of molecules consisting
largely of carbon, hydrogen, nitrogen, oxygen, phosphorus, and sulfur. |
||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||
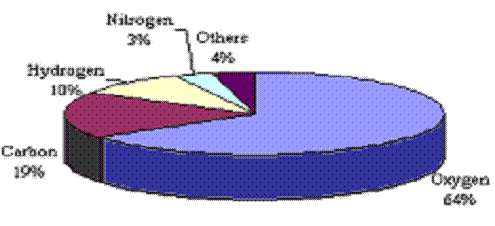
Naturally Occurring Elements in the Human Body |
||||||||||||||||||||||||||||||||||||||||||||||||
From Cummings et al. Table 2.1, Biology. 2001. |
||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
The elements in your body. (Adapted From Cummings et al. Table 2.1, Biology. 2001.) |
||||||||||||||||||||||||||||||||||||||||||||||||
|
More than a hundred different elements have been discovered so far. The elements that occur in nature are called the natural elements. Some natural elements are oxygen, calcium, nitrogen, and zinc. Synthetic elements have been made by scientists in the laboratory. When scientists discovered a synthetic element, they had the privilege of naming it. Californium was discovered at the University of Berkeley in California. Plutonium was named after the planet Pluto. |
||||||||||||||||||||||||||||||||||||||||||||||||
Organic Elements
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
The organic elements are those elements commonly found in living organisms. They compose the building blocks for our organic molecules, like proteins, sugars, fats, and genetic material. These elements also consist of ions needed for common organic processes, like nerve cells communicating with each other, moving muscles, or releasing adrenaline. The most common organic elements are oxygen, carbon, hydrogen and nitrogen. Together, these atoms form 96.3% of the Human body by weight. The following table shows the most commonly occurring natural elements found in the Human Body. |
||||||||||||||||||||||||||||||||||||||||||||||||
|
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||
|
c. Living organisms have many different kinds of
molecules, including small ones, such as water and salt, and very large ones,
such as carbohydrates, fats, proteins, and DNA.
|
|
Organic
Groups |
|
As they form organic molecules, elements are
commonly arranged in organic groups. There are six common organic groups
that for smaller units of organic molecules. These are methyl, hydroxyl,
carboxyl, amino, sulfydrl, and phosphate groups. By combing these groups in
particular arrangements, common organic molecules are formed. |
Organic Molecules |
There
are four basic groups of organic molecules:
Proteins, Carbohydrates, Lipids, and Nucleic Acids.
These molecules are made by
bonding different organic groups to each other in differing orders.
|
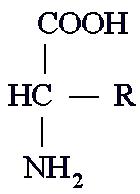
All organic molecules contains methyl groups, and most contain hydroxyl groups.
For example, you can describe the basic amino acid, the molecule that
makes proteins, as a molecule that contains a methyl group, an amine group,
and a carboxyl group, plus one R-group that varies from amino acid to amino
acid. Carbohydrates have 3-6 methyl groups, with about the same number of
hydroxyl groups, and maybe containing a carboxyl group. Lipids are strings of
methyl groups, with one carboxyl group at one end. Nucleic Acids have
alternating methyl and amine groups.
|
Proteins: |
Elements: C, H, O, N, and sometimes S.
|
Function: Enzymes, structural proteins, storage proteins,
transport proteins, hormones, proteins for movement, protection, and toxins.
|
General Structure |
|
There are 20+ amino acids, each differing
only in the composition of the R groups. An R group could be a sulfydrl,
another methyl, a string a methyls, rings of carbons, and several other
organic groups. Proteins can be either acidic or basic, hydrophilic or
hydrophobic. The following table shows 20 amino acids that common in
proteins. |
Carbohydrates: |
||
|
Elements: C, H, and O. |
||
|
Function: Energy, structure |
||
|
Carbohydrates are sugars and starches.
The most basic structure consist of 3-6 carbons, but we are going to concentrate
upon sugars that form a either a pentagon ring (5-carbon sugars) or a hexagon
ring (6-carbon sugars). These sugars are named pentoses and hexoses
respectively. The sufix –oses refers to sugar, and prefix refers to the
number of carbons. One corner of the ring has an oxygen, so that one carbon
group lies outside of the ring. Attached to each carbon is a hydroxyl group,
and a hydrogen. If you said that carbohydrates had a primary structure, akin
to proteins, it would be the order of the sugars, the pentoses and the
hexoses. The simplest sugars are monosaccharides (mono–: one;
–saccaharides: sugar). Among the hexoses, sugars having six
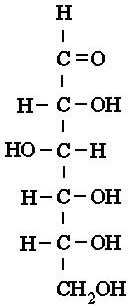
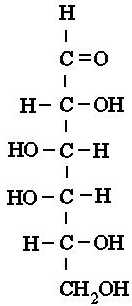
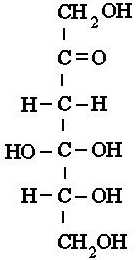
carbons, there are glucose, galactose, and fructose. Both glucose and
galactose have very similar structures, and only differ in the arrangement of
on hydroxyl group on the 4th carbon. Fructose looks more like
glucose than galactose, but it differs from glucose by having a hydroxyl
group on the 1st carbon, with its 2nd carbon having the
double bond with oxygen |
||
|
|
|
|
|
Glucose |
Galactose |
Fructose |
|
Above: Simple diagrams of
glucose, galactose, and fructose not showing the hexagon shape. |
||
Lipids: |
|||||||||||||||||
|
Elements: C, H, and O. |
|||||||||||||||||
|
Function: Storage, cushion, hormones. |
|||||||||||||||||
Fatty Acids:
|
|||||||||||||||||
|
Fatty acids are lipids that are made from long chains of
methyls. Fatty acids can be either saturated, where the chain only has
groups of CH2, or fatty acids can be unsaturated, where
there are one or more CH = CH groups, carbons attached with a double bond to
another carbon. Think of the fatty acids as being unsaturated with H, since
to form a double bond, two carbons must lose H. So saturated fatty acids are
saturated with H, and unsaturated fatty acids have room for more H atoms. At room
temperature, saturated fatty acids are waxy solids, and unsaturated fatty
acids are liquid. Below are two 18C fatty acids, stearic acid and oleic acid.
They differ only in that stearic acid is saturated with H, while oleic acid
is an unsaturated fatty acid. |
|||||||||||||||||
|
|
|||||||||||||||||
|
Stearic
acid |
|||||||||||||||||
|
Common Fatty
Acids |
|||||||||||||||||
|
|||||||||||||||||
|
Triglycerides: |
|
||||||||||||||||
|
Triglycerides, or fats, have the simplest
form of all lipids. In plants, triglycerides form the major proportion of
lipids in plants. In animal, adipose cells (fat cells) stores triglycerides
for future use as energy. Triglycerides are made from three chains of fatty
acids, bonded to a pole of glycerol. In the molecular formula below, the
R-group represents fatty acids, where they can either be all different, be
the same, or only two fatty acids be the same. |
|
||||||||||||||||
|
Essential Fatty Acids: |
|||||||||||||||||
|
Just as there
are essential amino acids that our bodies can not synthesis, there are also
essential fatty acids, like linoleic acid, that our body has to get our from food.
We can easily make saturated fatty acids and unsaturated fatty acids that
have one double bond, but we do not have the proper enzymes to synthesis
unsaturated fatty acids that have more than one double bond. These fatty
acids are very important to our immune system and to help us regulate our
blood pressure, for they are used to make essential compounds, such as
prostaglandins. Prostaglandins are a group of organic molecular messengers
that changes our blood pressure, open air passages, and cause uterine
contraction. |
|||||||||||||||||
Nucleic Acids: |
|||||||
|
Elements: C, H, O, and N. |
|||||||
|
Function: Blueprint for protein synthesis in cells, heredity. |
|||||||
|
Of the organic molecules, there are fewer
nucleic acids, yet they the most unique parts among the organic molecules.
Nucleic acids are made from three organic groups, a phosphate group, a
pentose sugar, and nitrogen bases. One nucleic acid is bound to
another through the phosphate group. Nucleic acids can be divided into two
groups, ribonucleic acid (RNA) and deoxyribonucleic acid (DNA).
|
|||||||
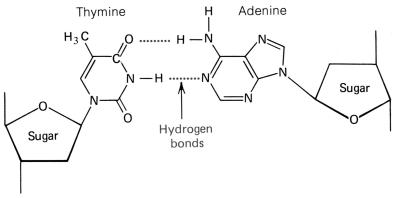
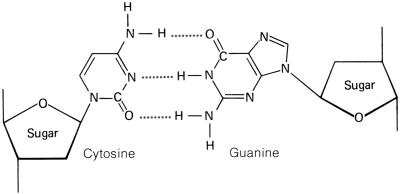
There are only three differences between
these two nucleic acids. First, RNA is made with the pentose sugar ribose,
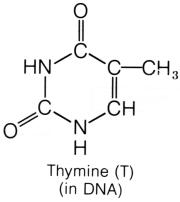
and DNA is made with the pentose sugar deoxyribose. Second, RNA
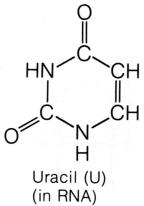
contains the nitrogen base uracil, and DNA contains the nitrogen base thymine.
Third, RNA has only one strand of nucleic acids, while DNA is made from a
double strand, wrapping around each other in a double helix. Beyond the
pentose sugar, nucleic acids differ in the nitrogen base that they
contain. The nitrogen bases can be divided into two groups based upon the
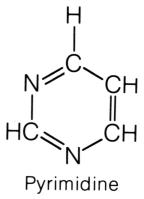
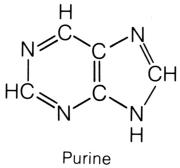
shape of the nitrogen base, pyrimidines and purines. The pyrimidines
have a hexagon shape, generally made with four carbons and two nitrogens. The
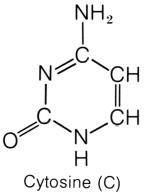
pyrimidine cytosine has an amine group attached to the first
carbon. Both thymine and uracil has an oxygen in place of the amine group.
But thymine has a methyl group attached to the second carbon, where uracil
does not. The pyrimidines are bonded to the pentose sugar by the nitrogen
that lies at the third position, between the CH and C=O, on the hexagon ring. The purines have a double-ring shape, with a pentagon attached
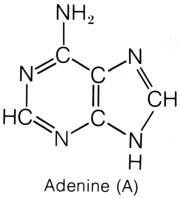
to one side of a hexagon. Adenine , similar to cytosine, has an amine
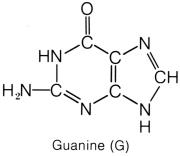
group attached to the first carbon of the hexagon ring. Guanine,
similar to thymine and uracil, has an oxygen in place of the amine group. The
purines are bonded to the pentose sugar by the nitrogen on the pentagon ring.
On DNA, and when RNA is being made, RNA
synthesis, pyrimidines attach to purines by hydrogen bonds between the
nitrogens of the purines, as well as the double-bonded oxygen and the
nitrogen on the pyrimidine.
It is the arrangement of the nucleic
acids on these large genetic molecules that form the genetic code for living
organisms. |
|
|
|||
|
Bd glucose |
|
|
|
Cholesterol |
4
types of Bio-Chemicals
Carbs
– Glucose (the sugar in your blood, sucrose (granulated sugar), fructose
(in honey, fruits)
Lipids(Fats/Oils)
–
Nucleic
Acids – DNA/RNA
Proteins
-
|
|
|
|
|
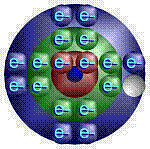
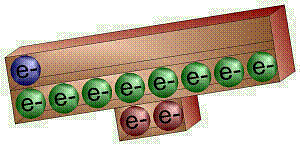
For
any element, the electrons in the outermost energy level/shell are the most
important. These determine an element’s chemical properties – how
it will react in a chemical reaction. These important electrons are known as
the valence electrons. Know how many valence electrons carbon, oxygen,
hydrogen, nitrogen, sodium, and chlorine have. |
|
|
|
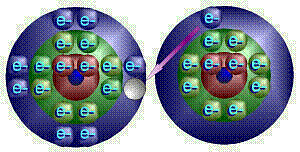
Thus,
when sodium and chlorine come together, in an explosive reaction, chlorine
grabs sodium’s “unwanted” electron. This forms sodium ions
with a +1 electrical charge (extra proton because it lost an electron) and
chloride ions with a –1 charge. Chemists would write this as Na + Cl |
|
Water |
|
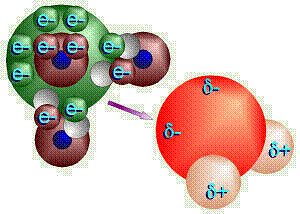
The
shape of a water molecule is also a tetrahedron. Oxygen has six valence
electrons and two “holes,” thus can bond with two hydrogens.
Therefore, the chemical formula for water is H2O. Oxygen’s
other four valence electrons, in two pairs, are not bonded to any other
atoms, thus these are referred to as unshared pairs of electrons.
Oxygen shares electrons with hydrogen, but pulls just a little harder on the
electrons. The electrons are just a little closer to the oxygen than the
hydrogens, so this is called a polar covalent bond. Note that even
though the molecule as a whole is electrically neutral (the + and –
charges balance), the ends of the molecule where the hydrogen nuclei are
(which contain only a proton) have a sort-of positive charge, and the ends of
the molecule by the unshared pairs of electrons are sort-of negative. The
sort-of positive ends on one water molecule are attracted to the sort-of
negative ends on another water molecule. This is called hydrogen bonding.
Actually, hydrogen bonding can happen with other molecules besides water as
we will see later. |
|
Water
is a key ingredient in all life. Cells are 70 to 95% water. About 75% of the
Earth's surface is covered with water. Water is the only common substance existing
naturally in all three forms: solid, liquid, gas. Water has many unique
properties due, in great part, to its hydrogen bonding. |
|
Water
sticks to itself. It forms droplets. It acts like it has a film on top. Water
sticks to other things; well, at least some other things. Hydrophilic substances
like glass, paper, and sugar can mix with or stick to water (yes, on very
clean glass, water “sheets” and flows over the glass without
beading up). On the other hand, hydrophobic
substances like teflon, salad oil, and car wax will not mix with water, and
water placed on those surfaces will bead up. Water can
absorb a lot of heat without changing temperature very much. No,
“heat” and “temperature” are not the same thing! For
example, suppose you have a Corningware® or glass pot and an
aluminum pot that weigh the same amount (contain the same amount of material).
If you would place these two pots on equal burners on the stove, which would
get hot faster? Aluminum, right? The Corningware pot can absorb more of the
heat from the burner without changing temperature as much. In the summer, in
the daytime, lakes and oceans can absorb a lot of heat from the sun, keeping
the surrounding air much cooler. At night and/or in the winter, bodies of
water will gradually give up their heat, warming the surrounding air.
That’s why costal areas tend to have more mild seasonal changes. In
deserts, where there is very little water to absorb and retain heat, summer
daytime temperatures can be extremely high, yet at night, a person would need
a warm jacket, campfire, and/or sleeping bag to keep warm in the 40 to
50° F (5 to 10° C) weather. This property of water is also useful in
our bodies: in the summer we can absorb a lot of heat from the sun without
overheating too much, and in the winter we don’t immediately freeze
when we go outside. Ice
floats. For most substances, the solid form is more dense than the liquid
form and would sink to the bottom of any mixture of the two, but for water,
the solid (ice) is less dense. If ice sank, in winter as water froze, it
would sink to the bottom of ponds and lakes, thus they would freeze from the
bottom up. In spring/summer, only the top few inches would thaw because the
solid ice on the bottom would never rise high enough to be warmed by the sun.
Thus, it would be impossible for any organisms to live in water. |
|
pH |
|
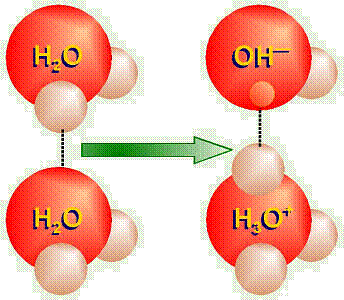
Even
in plain, distilled water, because of the hydrogen bonding, sometimes one of
the hydrogen protons from one water molecule “jumps over” to one
of the pairs of unshared electrons in another water molecule (leaving its
electron behind). Thus ions of H3O+ (hydronium ion) and
OH– (hydroxide ion) are formed. This reaction would be
written as 2H2O
f
other substances are added, the concentrations of hydrogen (hydronium) and
hydroxide ions (notated as [H+] and [OH–]) may
change, but pH is always based on the hydrogen ion concentration, [H+].
If [H+] is greater than 0.0000001 M, (like 0.0001 or 10–4
so pH = 4), that solution is an acid, and if [H+] is less than
0.0000001 M, (like 0.0000000001 or 10–10 so pH = 10)
the solution is a base. Somebody figured out that [H+] × [OH–]
always equals 10–14, so if one increases, the other
decreases, proportionately, such that the product of the two will always be
10–14. |
|
ACIDS & BASES |
|
An
acid is a substance which adds H+ to a solution. A base
is a substance which subtracts H+ from or adds OH–
to a solution. A neutral solution has a pH of 7. If the pH of a solution is
less than 7 (because [H+] is greater than 10–7)
the solution is an acid, and if the pH is greater than 7 (because [H+]
is less than 10–7) the solution is a base. Biological
substances like lemon juice and vinegar are acids, and both of these have pH
values around 3. The hydrochloric acid (HCl) in toilet bowl cleaner and in
our stomachs is a strong acid — the pH of stomach acid is between 1 and
3. Lye (NaOH), the main ingredient in many drain-openers, is a strong base,
with a pH of 12 to 14, depending on the concentration of the solution. I have
heard that the typical pH of our scalp and skin is around 5, slightly acidic,
and that pH is best for skin and scalp health and resistance to infection and
diseases. Soap and many shampoos are made via a chemical reaction involving
lye, and since there is typically a bit of unreacted lye left in them, they
are bases (some quite strong bases). Thus, while our skin can secrete
chemicals to recover its pH balance following occasional, reasonable use of
these products, too-frequent use of these products (shampooing one’s
hair on a daily basis, daily showers using soap or detergent bars) can prevent
the scalp/skin from maintaining a normal pH, resulting in “dry”
(= less healthy) skin and an increased risk of infection. Thus, some shampoo
manufacturers add chemicals to their shampoos to lower the pH closer to the
skin’s normal of pH 5, and these shampoos are often marketed as
“pH-balanced” shampoos. I have heard knowledgeable people,
including dermatologists, say that it is better for one’s skin to not
take daily showers, or at least to not use soap (just rinse with water) if
someone feels that a daily shower is a “must.” (Interestingly,
while I have not seen actual scientific studies on this, I have heard/read
that our scalp secretes chemicals which help to repel head lice, and the
suggestion that the increased incidence of head lice among school children in
our country may, in part, be related to the fact that parents are actually
keeping their children’s hair “too clean,” thereby
preventing the accumulation of an adequate supply of natural repellant on
their hair.) |
|
Buffers |
|
A
buffer is a substance which minimizes the change in pH or [H+].
Different buffers work best at different pH ranges. Notice that what’s
happening here is that a buffer protects from too great of a change in
pH. By no means is this anything like the equivalent of lowering/minimizing
the pH, which would have the effect of creating a strong acid. The concept of
pH and the utilization of buffers to maintain “normal” pH ranges
are important in our bodies and in other branches of in biology. For example,
an enzyme called pepsin
digests protein in our stomachs, but must have an acid environment to
function (most of the enzymes in our bodies only function within certain pH
ranges). Not all of the food we eat is acidic, and might destroy (or neutralize)
the normal stomach pH, thus making the pepsin ineffective, unable to digest
dietary protein. To prevent this from happening, the buffers in our
stomach keep the pH fairly constant, within a range of about pH 1 to 3.
However, antacids such as Tums® or Rolaids® are
so “strong” that they overwhelm the person’s
stomach’s buffers’ ability to function properly, drastically
changing the pH of the stomach contents, and therefore, pepsin’s
ability to digest the protein in one’s diet. Calcium, by the way, is
absorbed better into one’s body if the stomach contents are acidic,
thus antacids also interfere with our bodies’ ability to properly
absorb calcium. To properly absorb dietary calcium, it should be consumed
along with acidic or slightly acidic substances (such as milk or orange
juice), and not mixed in with antacids. While there may be legitimate uses
for antacids (such as when a doctor prescribes them to assist in treating
ulcers), frequent, “casual” use of antacids may actually stimulate
the production of more stomach acid as the user’s system
struggles to overcome them and return the body to normal. As another example,
our blood must remain very close to around pH 7.4, and if it deviates too
much, a person could get very sick or die. Yet, when we transport carbon
dioxide from our cells to our lungs, it turns into carbonic acid in the
deoxygenated blood. Thus, if it weren’t for buffers, our blood would be
a drastically different pH depending on how much carbon dioxide was dissolved
in it. |
|
|
|
|
|
|
References:
SAS
Chem – Elements
http://www.starsandseas.com/SAS%20OrgChem/SASElements.htm
Library
of PDB files for molecules alphabetically
http://www.wellesley.edu/Chemistry/Flick/molecules/newlist.html
Organic Building Blocks Lab
Background - Organic Molecules
There are four basic groups of organic molecules: Proteins,
Carbohydrates, Lipids, and Nucleic Acids. These molecules
are made by bonding different organic groups to each other in differing orders.
All organic molecules
contains methyl groups, and most contain hydroxyl groups. For example, you can describe
the basic amino acid, the molecule that makes proteins, as a molecule that
contains a methyl group, an amine group, and a carboxyl group, plus one R-group
that varies from amino acid to amino acid. Carbohydrates have 3-6 methyl
groups, with about the same number of hydroxyl groups, and maybe containing a
carboxyl group. Lipids are strings of methyl groups, with one carboxyl group at
one end. Nucleic Acids have alternating methyl and amine groups.
|
Organic building blocks |
||
|
Organic Group |
Formula |
Compounds |
|
1. Methyl |
—CH3 and —CH2 |
proteins, carbohydrates, lipids, nucleic acids, etc. |
|
2. Hydroxyl |
—OH |
alcohol |
|
3. Carboxyl |
—COOH and —COO– |
proteins, lipids |
|
4. Amino |
—NH2 |
proteins, amino acids, nucleic acids |
|
5.:Sulfydrl |
—SH |
some amino acids, Thiols |
|
6. Phosphate |
—PO4 |
organic phosphates like ATP, DNA, RNA |
For
this lab you are going to use the molecular modeling kits to investigate how
many ways a group of Carbon atoms may
be bonded together, what the 6 organic building blocks are, what the essential
components of organic compounds are, and what distinguishes the 4 main groups
of organic compounds from each other.
STEP 1: Explore Carbon Bonding
Bond
5 Carbon atoms to each other as many ways as you can. You may use single,
double, and triple bonds.
DRAW
each configuration in your LAB NOTES.
STEP
2: BUILDING BLOCK CREATION
Create
_____ Methyl, ____ Hydroxyl, ____ Carboxyl, ____ Amino, ____ Sulfydrl, and ____
Phosphate structures.
DRAW
a picture of one molecule for each of these in your LAB NOTES.
STEP
3: BUILD AN ORGANIC MOLECULE
Using
the structures created in STEP 2 build a _________________ molecule.
DRAW
a picture of it in your LAB NOTES.
STEP
4: BUILD ANOTHER TYPE OF ORGANIC MOLECULE
Using
the remaining building blocks build a __________________molecule.
DRAW
a picture of it in your LAB NOTES.
STEP
5: TRADE or SWAP TO BUILD YOUR LAST ORGANIC MOLECULE
Now
look at the remaining structures you have determine what you need to build a
___________________ molecule. On the bid cards write what structure you need
and which one you would trade (you don’t need it) and pass the card to
the next group to see if you can arrange a trade to complete your molecule.
DRAW
the resulting molecule in your LAB NOTES.
Raise your hand to show rest of class your final molecules.
LAB QUESTIONS:
1. How many configurations of the Carbon atoms did you make?
2. What are the 3 most common elements used in the models you built for this exercise?
3. Based on what you already know about acids and bases, are alcohols typically acids or bases?
4. Could you create any of these organic molecules without Carbon? YES/NO
5. If yes to #4, list two.
6. Could you create any of these organic molecules without Oxygen?
7. If yes to #6, list two.
8. Could you create any of these organic molecules without Hydrogen?
9. If yes to #8, list two.
10. Do you understand organic compounds better now?
5.
CARBOHYDRATES
|
|
|
|
|
Glucose |
Galactose |
Fructose |
![[Formation of Sodium Chloride]](organihandout_files/image002.gif)
![[Formation of Methane]](organihandout_files/image004.gif)
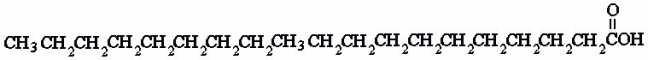
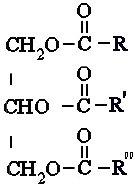
![[Periodic Table]](organihandout_files/image034.gif)
![[Sodium's Electron Orbitals]](organihandout_files/image036.gif)