| |
|
WATER |
H2O |
YRY |
| |
|
|
| Hydrochloric Acid |
HCl |
|
| |
|
|
| Methane |
CH4 |
|
 |
|
|
| Carbon Dioxide |
CO2 |
|
| |
|
|
| METHANOL (Wood Alcohol) |
CH3OH |
|
 |
|
|
| ETHANOL (ethyl alcohol) |
CH2OH |
|
| |
|
|
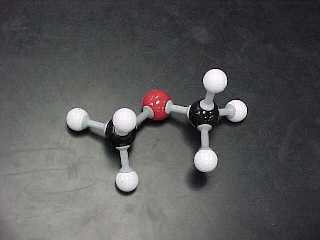
| DIMETHYL ETHER |
CH3OCH3 |
|
 |
|
|
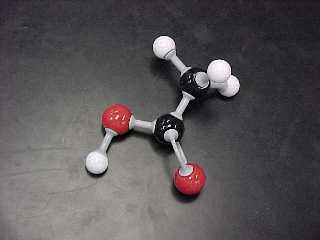
| ETHANOIC ACID(also known as acetic acid) |
CH3COOH |
|
 |
|
|
| CARBONIC ACID |
CH2O2 |
|
| |
|
|
| VINYL CHLORIDE |
C2H3Cl |
|
| |
|
|
| GLUCOSE (Sugar) |
C6H12O6 |
|
| |
|
|
| ISOMERIC BUTANE |
C4H10 |
|
 |
|
|
| |
|
|
| |
|
|
| ETHENE(also called acetylene) |
,C2H2
|
|
 |
|
|
| CARBON TETRA CHLORIDE |
|
|
| |
|
|
| POTASSIUM CHLORIDE |
|
|
| |
|
|
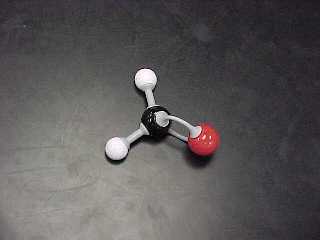
| METHANAL (A.K.A. Formaldehyde) |
CH2O |
|
 |
|
|
| |
|
|
| |
|
|
|
 |
|
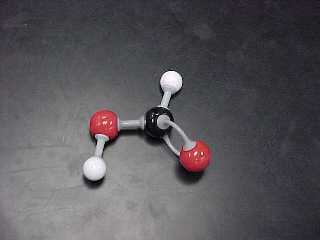
methanoic acid (also known as formic acid) |
|
HCOOH |
|
|
ethane, C2H6
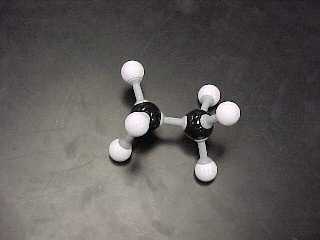 |
|
propane, C3H8
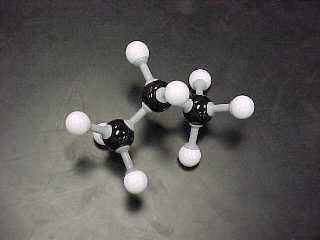 |
butane, C4H10
&
pentane, C5H12
 |
|
hexane, C6H14
& eptane, C7H16
 |
|
|
Special Note: there are
TWO (or more) different ways to make
some structures, starting with the C4H10
formula. |
|
ethyne (also called acetylene), C2H2
 |
propyne, C3H4
 |
|
butyne, C4H6
 |
|
Special Note: Butyne can have the triple
bond in two different locations. |
|